Genital Warts Treatment
Genital Warts
Of genital warts, 90% are caused by HPV 6 or 11. HPV types 6 or 11 are commonly found before, or at the time of, detection of genital warts (406).
HPV types 16, 18, 31, 33, and 35 are found occasionally in visible genital warts (usually as coinfections with HPV 6 or 11) and can be associated with foci of high-grade intraepithelial neoplasia, particularly in persons who are infected with HIV infection.
In addition to warts on genital areas, HPV types 6 and 11 have been associated with conjunctival, nasal, oral, and laryngeal warts.
Signs and symptoms
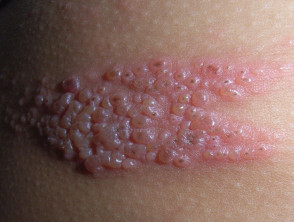
Genital warts are usually asymptomatic, but depending on the size and anatomic location, they can be painful or pruritic. Genital warts are usually flat, papular, or pedunculated growths on the genital mucosa.
Genital warts occur commonly at certain anatomic sites, including around the introitus in women, under the foreskin of the uncircumcised penis, and on the shaft of the circumcised penis.
Genital warts can also occur at multiple sites in the anogenital epithelium or within the anogenital tract (e.g., cervix, vagina, urethra, perineum, perianal skin, and scrotum). Intra-anal warts are observed predominantly in persons who have had receptive anal intercourse, but they can also occur in men and women who do not have a history of anal sexual contact.
Diagnosis of genital warts
Diagnosis of genital warts is usually clinical, made by visual inspection. Genital warts can be confirmed by biopsy, which might be indicated if 1) the diagnosis is uncertain; 2) the lesions do not respond to standard therapy; 3) the disease worsens during therapy; 4) the lesion is atypical; 5) the patient has comprised immunity; or 6) the warts are pigmented, indurated, fixed, bleeding, or ulcerated. Genital warts are usually asymptomatic, but depending on the size and anatomic location, they might be painful or pruritic. The use of HPV DNA testing for genital wart diagnosis is not recommended, because test results would not alter clinical management of the condition.
The application of 3%–5% acetic acid, which causes skin color to turn white, has been used by some providers to detect HPV-infected genital mucosa. However, acetic acid application is not a specific test for HPV infection. Therefore, the routine use of this procedure for screening to detect mucosal changes attributed to HPV infection is not recommended.
Treatment
The primary reason for treating genital warts is the amelioration of symptoms (including relieving cosmetic concerns) and ultimately, removal of the warts. In most patients, treatment can induce wart-free periods. If left untreated, visible genital warts can resolve on their own, remain unchanged, or increase in size or number. Available therapies for genital warts likely reduce, but probably do not eradicate, HPV infectivity. Whether the reduction in HPV viral DNA resulting from treatment reduces future transmission remains unclear. No evidence indicates that the presence of genital warts or their treatment is associated with the development of cervical cancer.
Regimens
Treatment of genital warts should be guided by the preference of the patient, available resources, and the experience of the health-care provider. No definitive evidence suggests that any of the available treatments are superior to any other, and no single treatment is ideal for all patients or all warts. The use of locally developed and monitored treatment algorithms has been associated with improved clinical outcomes and should be encouraged. Because of uncertainty regarding the effect of treatment on future transmission of HPV and the possibility of spontaneous resolution, an acceptable alternative for some persons is to forego treatment and wait for spontaneous resolution.
Factors that influence selection of treatment include wart size, wart number, anatomic site of the wart, wart morphology, patient preference, cost of treatment, convenience, adverse effects, and provider experience. Factors that might affect response to therapy include the presence of immunosuppression and compliance with therapy, which can consist of either a single treatment or complete course of treatment. In general, warts located on moist surfaces or in intertriginous areas respond best to topical treatment. The treatment modality should be changed if a patient has not improved substantially after a complete course of treatment or if side effects are severe. Most genital warts respond within 3 months of therapy. The response to treatment and any side effects should be evaluated throughout the course of therapy.
Complications occur rarely when treatment is administered properly. Patients should be warned that persistent hypopigmentation or hyperpigmentation occurs commonly with ablative modalities and has also been described with immune modulating therapies (imiquimod). Depressed or hypertrophic scars are uncommon but can occur, especially if the patient has had insufficient time to heal between treatments. Rarely, treatment can result in disabling chronic pain syndromes (e.g., vulvodynia and hyperesthesia of the treatment site) or, in the case of anal warts, painful defecation or fistulas. A limited number of case reports of severe systemic effects resulting from treatment with podophyllin resin and interferon have been documented.
Treatment regimens are classified into patient-applied and provider-applied modalities. Patient-applied modalities are preferred by some patients because they can be administered in the privacy of the patient’s home. To ensure that patient-applied modalities are effective, patients must comply with the treatment regimen and must be capable of identifying and reaching all genital warts. Follow-up visits are not required for persons using patient-applied therapy. However, follow-up visits after several weeks of therapy enable providers to answer any questions patients might have about the use of the medication and any side effects they have experienced; follow-up visits also facilitate the assessment of a patient’s response to treatment.
Recommended Regimens for External Genital Warts
Patient-Applied:
Podofilox 0.5% solution or gel
OR
Imiquimod 5% cream
OR
Sinecatechins 15% ointment
Provider–Administered:
Cryotherapy with liquid nitrogen or cryoprobe. Repeat applications every 1–2 weeks.
OR
Podophyllin resin 10%–25% in a compound tincture of benzoin
OR
Trichloroacetic acid (TCA) or Bichloroacetic acid (BCA) 80%–90%
OR
Surgical removal either by tangential scissor excision, tangential shave excision, curettage, or electrosurgery.
Podofilox
Podofilox is an antimitotic drug that destroys warts, is relatively inexpensive, easy to use, safe, and self-applied. Podofilox solution should be applied with a cotton swab, or podofilox gel with a finger, to visible genital warts twice a day for 3 days, followed by 4 days of no therapy. This cycle can be repeated, as necessary, for up to four cycles.
The total wart area treated should not exceed 10 cm2, and the total volume of podofilox should be limited to 0.5 mL per day. If possible, the health-care provider should apply the initial treatment to demonstrate the proper application technique and identify which warts should be treated. Mild to moderate pain or local irritation might develop after treatment.
The safety of podofilox during pregnancy has not been established.
Imiquimod
Imiquimod is a topically active immune enhancer that stimulates production of interferon and other cytokines. Imiquimod cream should be applied once daily at bedtime, three times a week for up to 16 weeks (407).
The treatment area should be washed with soap and water 6–10 hours after the application. Local inflammatory reactions, including redness, irritation, induration, ulceration/erosions, and vesicles, are common with the use of imiquimod, and hypopigmentation has also been described (408). Imiquimod might weaken condoms and vaginal diaphragms. The safety of imiquimod during pregnancy has not been established.
Sinecatechin ointment
Sinecatechin ointment, a green-tea extract with an active product (catechins), should be applied three times daily (0.5-cm strand of ointment to each wart) using a finger to ensure coverage with a thin layer of ointment until complete clearance of warts. This product should not be continued for longer than 16 weeks (409–411).
The medication should not be washed off after use. Sexual (i.e., genital, anal, or oral) contact should be avoided while the ointment is on the skin. The most common side effects of sinecatechins 15% are erythema, pruritis/burning, pain, ulceration, edema, induration, and vesicular rash.
This medication may weaken condoms and diaphragms. No clinical data are available regarding the efficacy or safety of sinecatechins compared with other available anogenital wart treatment modalities.
The medication is not recommended for HIV-infected persons, immunocompromised persons, or persons with clinical genital herpes because the safety and efficacy of therapy in these settings has not been established. The safety of sinecatechins during pregnancy also is unknown.
Cryotherapy
Cryotherapy destroys warts by thermal-induced cytolysis. Health-care providers must be trained on the proper use of this therapy because over- and undertreatment can result in complications or low efficacy. Pain after application of the liquid nitrogen, followed by necrosis and sometimes blistering, is common. Local anesthesia (topical or injected) might facilitate therapy if warts are present in many areas or if the area of warts is large.
Podophyllin resin
Podophyllin resin 10%–25% should be applied to each wart and allowed to air-dry before the treated area comes into contact with clothing; overapplication or failure to air dry can result in local irritation caused by spread of the compound to adjacent areas.
The treatment can be repeated weekly, if necessary. To avoid the possibility of complications associated with systemic absorption and toxicity, two guidelines should be followed: 1) application should be limited to <0.5 mL of podophyllin or an area of <10 cm2 of warts per session and 2) the area to which treatment is administered should not contain any open lesions or wounds. The preparation should be thoroughly washed off 1–4 hours after application to reduce local irritation.
The safety of podophyllin during pregnancy has not been established. Podophyllin resin preparations differ in the concentration of active components and contaminants. The shelf life and stability of podophyllin preparations are unknown.
Trichloroacetic acid (TCA) or Bichloroacetic acid (BCA)
Both TCA and BCA are caustic agents that destroy warts by chemical coagulation of proteins. Although these preparations are widely used, they have not been investigated thoroughly.
TCA solutions have a low viscosity comparable with that of water and can spread rapidly if applied excessively; therefore, they can damage adjacent tissues. A small amount should be applied only to the warts and allowed to dry before the patient sits or stands, at which time a white frosting develops.
If pain is intense, the acid can be neutralized with soap or sodium bicarbonate. If an excess amount of acid is applied, the treated area should be powdered with talc, sodium bicarbonate (i.e., baking soda), or liquid soap preparations to remove unreacted acid. This treatment can be repeated weekly, if necessary.
Surgical therapy
Surgical therapy has the advantage of usually eliminating warts at a single visit. However, such therapy requires substantial clinical training, additional equipment, and a longer office visit.
After local anesthesia is applied, the visible genital warts can be physically destroyed by electrocautery, in which case no additional hemostasis is required. Care must be taken to control the depth of electrocautery to prevent scarring. Alternatively, the warts can be removed either by tangential excision with a pair of fine scissors or a scalpel, by laser, or by curettage.
Because most warts are exophytic, this procedure can be accomplished with a resulting wound that only extends into the upper dermis. Hemostasis can be achieved with an electrocautery unit or a chemical styptic (e.g., an aluminum chloride solution). Suturing is neither required nor indicated in most cases if surgical removal is performed properly.
Surgical therapy is most beneficial for patients who have a large number or area of genital warts. Both carbon dioxide laser and surgery might be useful in the management of extensive warts or intraurethral warts, particularly for those persons who have not responded to other treatments.
Because all available treatments have shortcomings, some clinics employ combination therapy (simultaneous use of two or more modalities on the same wart at the same time). Data are limited regarding the efficacy or risk of complications associated with use of such combinations.
Alternative Regimens
Alternative regimens include treatment options that might be associated with more side effects and/or less data on efficacy. Alternative regimens include intralesional interferon, photodynamic therapy, and topical cidofovir.
Recommended Regimen for Cervical Warts
For women who have exophytic cervical warts, a biopsy evaluation to exclude high-grade SIL must be performed before treatment is initiated. Management of exophytic cervical warts should include consultation with a specialist.
Recommended Regimens for Vaginal Warts
Cryotherapy with liquid nitrogen. The use of a cryoprobe in the vagina is not recommended because of the risk for vaginal perforation and fistula formation.
OR
TCA or BCA 80%–90% applied to warts. A small amount should be applied only to warts and allowed to dry, at which time a white frosting develops. If an excess amount of acid is applied, the treated area should be powdered with talc, sodium bicarbonate, or liquid soap preparations to remove unreacted acid.
This treatment can be repeated weekly, if necessary.
Recommended Regimens for Urethral Meatus Warts
Cryotherapy with liquid nitrogen
OR
Podophyllin 10%–25% in compound tincture of benzoin. The treatment area and adjacent normal skin must be dry before contact with podophyllin. This treatment can be repeated weekly, if necessary.
Data are limited on the use of podofilox and imiquimod for treatment of distal meatal warts.
Recommended Regimens for Anal Warts
Cryotherapy with liquid nitrogen
OR
TCA or BCA 80%–90% applied to warts. A small amount should be applied only to warts and allowed to dry, at which time a white frosting develops. If an excess amount of acid is applied, the treated area should be powdered with talc, sodium bicarbonate, or liquid soap preparations to remove unreacted acid. This treatment can be repeated weekly, if necessary.
OR
Surgical removal
Intra-anal warts should be managed in consultation with a specialist. Many persons with warts on the anal mucosa also have warts on the rectal mucosa, so persons with anal and/or intra-anal warts might benefit from an inspection of the rectal mucosa by digital examination, standard anoscopy, or high-resolution anoscopy.
Special Considerations
Pregnancy
Imiquimod, sinecatechins, podophyllin, and podofilox should not be used during pregnancy. Genital warts can proliferate and become friable during pregnancy. Although removal of warts during pregnancy can be considered, resolution might be incomplete or poor until pregnancy is complete. Rarely, HPV types 6 and 11 can cause respiratory papillomatosis in infants and children, although the route of transmission (i.e., transplacental, perinatal, or postnatal) is not completely understood. Whether cesarean section prevents respiratory papillomatosis in infants and children also is unclear (412); therefore, cesarean delivery should not be performed solely to prevent transmission of HPV infection to the newborn. Cesarean delivery is indicated for women with genital warts if the pelvic outlet is obstructed or if vaginal delivery would result in excessive bleeding. Pregnant women with genital warts should be counseled concerning the low risk for warts on the larynx (recurrent respiratory papillomatosis) in their infants or children.
HIV Infection
Persons who are HIV-infected are more likely to develop genital warts than persons who are not HIV-infected (413); moreover, lesions are more recalcitrant to treatment due to depressed cell-mediated immunity. No data suggest that treatment modalities for external genital warts should be different for HIV-infected persons. However, persons who are immunosuppressed because of HIV or other reasons might have larger or more numerous warts, might not respond as well as immunocompetent persons to therapy for genital warts, and might have more frequent recurrences after treatment (414–416). Squamous cell carcinomas arising in or resembling genital warts might occur more frequently among immunosuppressed persons, therefore requiring biopsy for confirmation of diagnosis for suspicious cases. Because of the increased incidence of anal cancer in HIV-infected MSM, screening for anal intraepithelial neoplasia by cytology can be considered (417). However, evidence is limited concerning the natural history of anal intraepithelial neoplasias, the reliability of screening methods, the safety and response to treatments, and the programmatic considerations that would support this screening approach.
Squamous Cell Carcinoma in Situ
Persons in whom squamous cell carcinoma in situ of the genitalia is diagnosed should be referred to a specialist for treatment. Ablative modalities usually are effective, but careful follow-up is essential for patient management.
Source
Genital Warts. Sexually Transmitted Diseases Treatment Guidelines, 2010
Centers for Disease Control and Prevention
GeoSalud, April 26, 2014
Related Articles
-
Human Papillomavirus
-
Human Papillomavirus Vaccines: Questions and Answers
-
Human Papilloma Virus Signs and Symptoms
-
Human Papillomavirus Screening
-
Human Papillomavirus Treatment
-
Human Papillomavirus Prevention
-
Human Papillomavirus and Men
-
HPV and Men
-
Human Papillomavirus Infection Treatment
-
Human Papillomavirus Vaccine
-
Human Papillomavirus and Genital Warts
-
Human Papillomavirus Associated Cancers
United States, 2004–2008 -
Preventing Cervical Cancer Worldwide (PDF document)
-
FDA Advisory Panel Recommends Human Papillomavirus Vaccine