Human Papillomavirus Vaccine. Reports of Health Concerns Following HPV Vaccination
Reports to VAERS Following Gardasil®
As of September 15, 2011, approximately 40 million doses of Gardasil® were distributed in the U.S. and VAERS received a total of 20,096 reports of adverse events following Gardasil® vaccination: 19,075 reports among females and 569 reports for males, of which 504 reports were received after the vaccine was licensed for males in October 2009.
VAERS received 452 reports of unknown gender. Of the total number of VAERS reports following Gardasil®, 92% were considered to be non-serious, and 8% were considered serious.
Non-serious adverse event reports
VAERS defines non-serious adverse events as those other than hospitalization, death, permanent disability, or life-threatening illness.
The vast majority (92%) of the adverse events reports following Gardasil® vaccination were mild and included, pain and swelling at the injection site (the arm), fever, dizziness, nausea, and fainting.
Syncope (fainting) is common after injections and vaccinations, especially in adolescents.
Falls after fainting may sometimes cause serious injuries, such as head injuries, which can be prevented by closely observing the person for 15 minutes after vaccination.
Serious adverse event reports
Any VAERS report that indicated hospitalization, permanent disability, life-threatening illness, congenital anomaly or death is classified as serious.
As with all VAERS reports, serious events may or may not have been caused by the vaccine.
Guillain-Barré Syndrome (GBS)
Guillain-Barré syndrome (GBS) has been reported after vaccination with Gardasil® .
GBS is a rare neurologic disorder that causes muscle weakness. It occurs in 1-2 out of every 100,000 people in their teens.
A number of infections have been associated with GBS.
There has been no indication that Gardasil® increases the rate of GBS above the rate expected in the general population, whether or not they were vaccinated.
Blood Clots
There have been some reports of blood clots in females after receiving Gardasil®.
These clots have occurred in the heart, lungs, and legs.
Most of these people had a risk of getting blood clots, such as taking oral contraceptives (the birth control pill), smoking, obesity, and other risk factors.
Deaths
As of September 15, 2011, there have been a total 71 VAERS reports of death among those who have received Gardasil®.
There were 57 reports among females, 3 were among males, and 11 were reports of unknown gender. Thirty four of the total death reports have been confirmed and 37 remain unconfirmed due to no identifiable patient information in the report such as a name and contact information to confirm the report.
A death report is confirmed (verified) after a medical doctor reviews the report and any associated records.
In the 34 reports confirmed, there was no unusual pattern or clustering to the deaths that would suggest that they were caused by the vaccine and some reports indicated a cause of death unrelated to vaccination.
VAERS Reports Following Cervarix®
Since licensed in October 2009, uptake of Cervarix® vaccination in the U.S. has been low.
As of September 2011, there have been 52 VAERS reports of adverse events following Cervarix® vaccination in the U.S.
The majority of these reports (98%) were considered to be non-serious.
Cervarix® has also been in use in other countries such as England and Europe prior to licensing from the FDA.
Summary
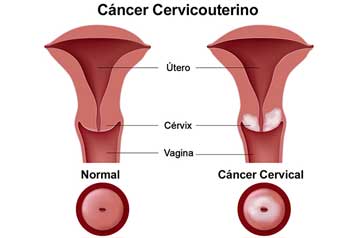
Based on all of the information we have today, CDC recommends HPV vaccination for the prevention of most types of cervical cancer.
As with all approved vaccines, CDC and FDA will continue to closely monitor the safety of HPV vaccines.
Any problems detected with these vaccines will be reported to health officials, healthcare providers, and the public and needed action will be taken to ensure the public's health and safety.
Source
Centers for Disease Control and Prevention
GeoSalud, january 23, 2014